WHY TO CHOOSE STAINLESS STEEL IN CONSTRUCTION
Stainless steel for rebar, fastening and anchoring applications
When does it make sense to work with stainless steel?
Reinforced concrete structures are not indefinitely durable and not free of maintenance costs. In cases where corrosion of the reinforcing steel is to be expected due to carbonation of the concrete or chloride input, additional corrosion protection measures are usually technically and economically reasonable. In the following cases, the use of stainless or rustproof steel is worthwhile:
- For the prevention of corrosion due to high chloride loads (e.g. in the case of de-icing salt parking decks, stairs and retaining walls along roads)
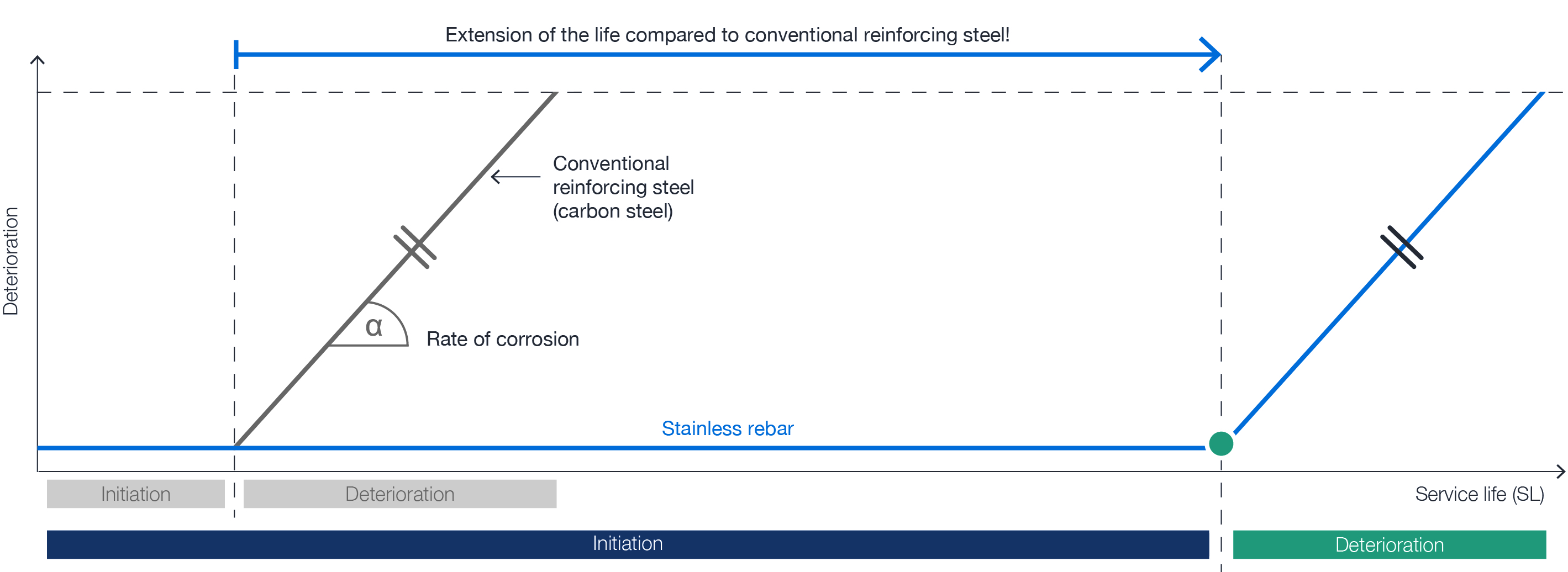
- In order to extend the service life of components and to reduce life cycle costs
- For the prevention of repairs (especially if inspectability is restricted)
- For fair faced concrete (architectural concrete) to avoid rust plumes
- For slimmer and lighter constructions through concrete cover reductions
Understanding stainless steel
Stainless steels are high-alloy steels with a chromium content of at least 10.5% and a maximum of 1.2% carbon. In normal environmental conditions and in aqueous neutral media, no surface corrosion and noticeable rust formation occur with these steels. A prerequisite for this behavior is a minimum content of certain alloying elements (e.g. chromium) in the steel and the presence of an oxidizing agent (e.g. oxygen) in the surrounding medium. Together this causes a passivation of the surface. Passivation means a strong inhibition of anodic iron dissolution after formation of passive layers on the surface. Such protective layers are very thin oxide layers. The passive layer inhibits any exchange between the metal and the surrounding medium with the result that these steels do not require any additional corrosion protection. In the event of damage to the passive layer (scratches, cuts, deformations, etc.), the passive layer reappears. This process is also known as "repassivation".


Understanding corrosion
| Exposure classes for rebar corrosion | Examples of environmental conditions | ||||
| No risk of attack | X0 | No risk of attack | Non-reinforced buildings (e.g. foundations withour rebar) | ||
| Carbonation induced rebar corrosion | XC1 | Dry or constantly wet | Interior components (incl. Kitchen, bathroom, components under water) | ||
| XC2 | Wet, rarely dry | Water tanks, foundations | |||
| XC3 | Moderate humidity | Exterior components, components regularly exposed to outside air | |||
| XC4 | Alternating wet and dry | Directly irrigated exterior components | |||
| Chloride induced rebar corrosion | XD1 | Moderate humidity | Spray mist areas of traffic surfaces, single car ports | ||
| XD2 | Wet, rarely dry | Swimming pools, spas, exposed to chloride water | |||
| XD3 | Alternating wet and dry | Spray water on deiced roads, parking decks | |||
| Chloride induced rebar corrosion from seawater | XS1 | Salty air, no direct contact with seawater | Exterior components in coastal areas | ||
| XS2 | Under water | Components in harbor areas, constantly submerged | |||
| XS3 | Tidal, sprayed water, spray mist areas | Quay walls, piers in harbors | |||
Concrete structures can be damaged by chemical or physical influences, resulting from the environmental conditions. Generally corrosion can be caused due to carbonation and due to chlorides, latter can result from e.g. de-icing salt or seawater. For the description of these influences, the European standard DIN 1045-1 has developed exposure classes. The classification of building structures into exposure classes combines, for example, requirements for the concrete cover and the concrete composition.
The relevant exposure classes for chloride induced corrosion are XD3 and XS3, due to high chloride loads; the relevant exposure classes for carbon induced corrosion are XC3 and XC4.
Please note, the mean surface chloride concentrations for XD3 can vary between 2.0 and 4.0 M.-%/z; based on empirical values, the upper limit for the surface chloride concentration may even be at 5.0 M.-%/z. In these highly exposed components even with the best concrete technology corrosion initiation will take place before the desired service life has been reached.
Chlorides on the outside of the concrete surface reach the reinforcement via transport processes. Above a certain chloride concentration at the level of the reinforcement (Ccrit = critical corrosion-initiating chloride content), the passive layer of the steel is destroyed and corrosion initiation occurs.
Depending on the corrosion resistance and thus essentially the alloy content of the reinforcement, corrosion will occur sooner or later. Besides the alloy content, the homogeneity of the steel surface has a decisive influence on the corrosion resistance.
This means that the level of corrosion resistance of reinforcing steels can be specifically influenced. From an economic point of view, the alloy content and the surface properties and thus the corrosion protection potential should be adapted to the expected effects.
Stainless steel facing the pitfalls of corrosion
Surface corrosion
The corrosion rate of stainless steels decreases with increasing pH value. In tap water or rainwater with a pH above 4, stainless steels behave passively. The pH value in damp concrete after carbonation is a minimum of 8, meaning stainless steel remains protected by its passivity.
For stainless steels, good resistance to surface corrosion and a certain susceptibility to pitting corrosion are characteristic. The presence of chlorides is decisive for triggering pitting corrosion. The probability of pitting corrosion occurring increases with increasing chloride concentration, increasing temperature and decreasing pH value. To a large extent the resistance to pitting depends on the surface and processing of the stainless steel. Ultimately with the right surface treatment the susceptibility of stainless steel for pitting corrosion can be significantly improved. Therefore Top12 is pickled in addition to the alloy content of 12% chrome.
Wherever possible, stainless reinforcing steel (e.g. Top12) is selectively used only in areas subject to chloride contamination. B500B is consistently used in areas without chloride exposure. This means that stainless reinforcing steel and B500B are often used in mixed reinforcement in the same component, which considerably reduces the quantities and the costs per component. Despite electrical contact between the more noble metal (stainless steel) and the less noble metal (B500B), contact corrosion (galvanic coupling) in the alkaline electrolyte (concrete) can be ruled out.
Choosing the most suitable steel grade for concrete constructions
| Produkt | Chloridkonzentration
[M.-%/z] in alkalischem Beton auf der Stahloberfläche |
Chloridkonzentration [M.-%/z] in karbonisiertem Beton auf der Stahloberfläche |
||||||||||||
| ≤ 0,5 | ≤ 1,0 | ≤ 2,0 | ≤ 2,5 | ≤ 3,0 | > 3,0 | ≤ 0,25 | ≤ 0,5 | ≤ 0,75 | ≤ 1,00 | ≤ 1,25 | > 1,50 | |||
| B500B | (1.4039) | |||||||||||||
| Top12 | (1.4003) | |||||||||||||
| UGIGRIP® | (1.4062; 1.4362; 1.4462) | |||||||||||||
| Korrosionsrisiko bei entsprechendem Produkt | keines | niedrig | hoch | |||||||||||
The comparative representation of the chloride resistances of the different concrete steels serves only as an overview. Decisive for the classification of the chloride resistance are the values for the critical corrosion-inducing chloride content (Ccrit), stated in expert opinions or comparable publications.
In order to improve reliability and durability of structures, while keeping in mind the overall costs, choosing the most suitable material is decisive. With an increasing amount of alloys, not only the durability of stainless steel increases, material costs increase as well. The target is to choose the steel reinforcement according to the corrosion exposure and the expected life-cycle of the structure. The following table shows different reinforcing steels classified according to their chloride resistance (Ccrit).
Take a look at a field study how reinforcements react to chlorides
Corrosion results after 12 years under real conditions in an Alps road tunnel (concrete layer 10mm)
Field tests in the Naxberg tunnel in Switzerland have shown impressively how different steels behave under high but common chloride loads from road traffic. Both the unalloyed reinforcing steel and the galvanized reinforcing steel exhibited considerable pitting corrosion and each had a corroded steel surface of over 70%. Top12 showed small rust spots, but no measurable material erosion could be determined for these spots. The duplex steel showed no signs of corrosion and was still passive after 12 years.
| Conventional rebar |
Top12
with rolling skin |
Galvanized rebar |
Duplex
steel 1.4462 |
|

|
||||